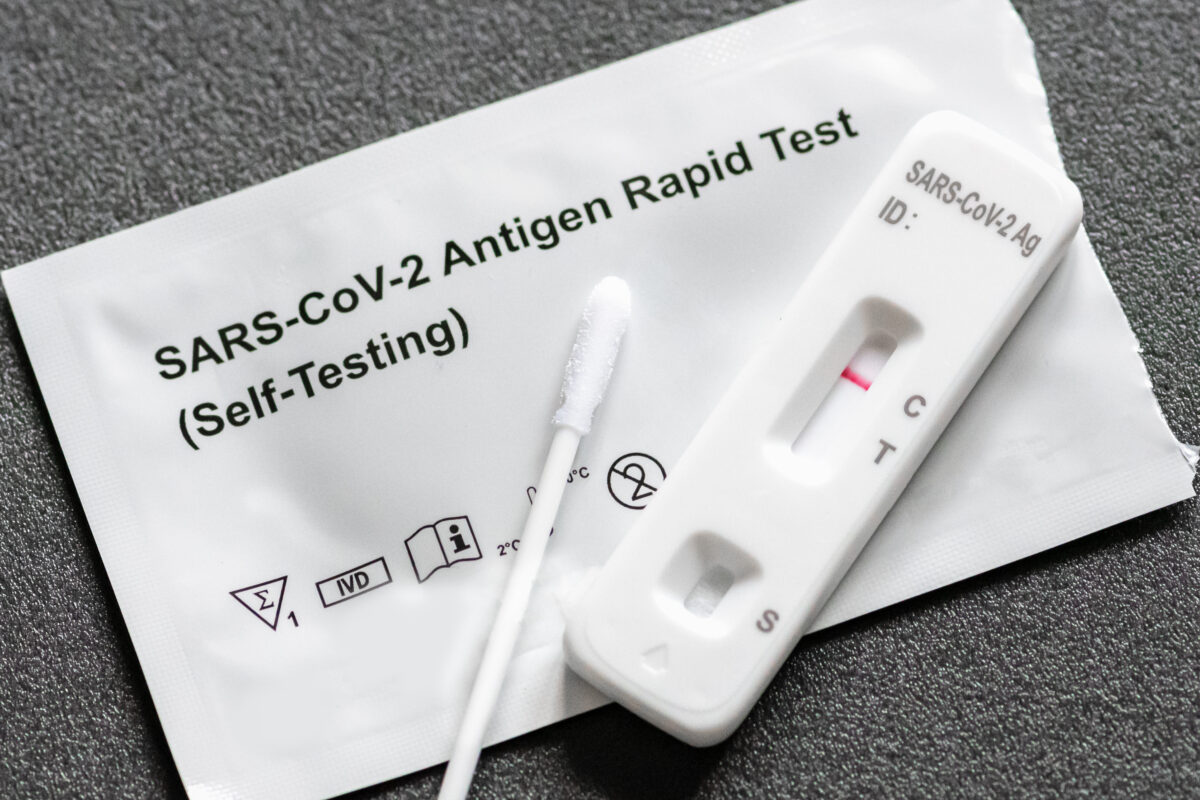
On Monday, Oct. 4, the U.S. Food and Drug Administration (FDA) authorized an at-home rapid COVID-19 test developed by ACON Laboratories, making the test the 11th of its kind to receive emergency use authorization. At-home rapid tests have received increased attention over the course of the pandemic, with the national COVID-19 response strategy placing new emphasis on their use and availability.
The first at-home rapid test received emergency use authorization from the FDA in November 2020. These tests are intended for convenient home use and do not require a prescription to purchase, with most producing test results in about 10‒15 minutes. Two popular brands of at-home rapid tests are the Abbott BinaxNOW Antigen Self Test and the Quidel QuickVue At-Home OTC COVID-19 Test.
At-home rapid tests work by detecting small virus particles, known as antigens, in a sample. Like other COVID-19 tests, these tests use a self-administered nasal swab to collect a sample from a person’s nasal passage. The swab is then combined with a chemical reactant to produce a test result.
At-home rapid tests are not as reliable as polymerase chain reaction (PCR) tests, which are usually administered in clinical or drive-thru testing sites and can identify a very small amount of virus particles in a sample. At-home rapid tests are less sensitive than PCR tests, and the potential for a false negative test is higher if a person tests too early in their infection. False negative results may result in spread of the disease if there is an assumption that a negative test always means that a person is not infected.
At-home rapid tests are most reliable when they are used on a person who has symptoms, because virus particles are less likely to be present in the nose of an infected person without symptoms. At-home tests can also be used for individuals who have been exposed to COVID-19, but it is best to wait three to five days after the exposure before testing.
Under President Joe Biden’s COVID-19 response plan announced in September, ramping up the at-home rapid COVID-19 testing is an important plan component. The Biden administration is asking test manufacturers to increase the production of at-home rapid tests and sell the tests at cost to customers. Additionally, the administration intends to send millions of rapid and at-home tests to local clinics and schools to monitor outbreaks more quickly. Accessibility of at-home rapid COVID tests has been a significant tool for infection control. For example, the United Kingdom provides a certain number of at-home rapid tests free of charge to citizens, Canada provides free tests to businesses, and in Germany at-home rapid tests are available for as little as $1.
While at-home rapid COVID tests provide a convenient option for testing, there is no requirement to report results of these tests to a centralized source. This presents challenges in public health efforts to monitor the extent of community spread, potentially undermining response efforts.