Authors
Pader Moua, MPH
Policy Analyst
501-526-2244
PMoua@achi.net
Arlo Kahn, MD
Medical Director
501-526-2244
KahnRonaldF@achi.net
(Update of post published July 28, 2022)
On September 1, the Centers for Disease Control and Prevention (CDC) announced updates to its COVID-19 booster recommendation, which replaced the original booster doses with a bivalent booster dose formulated to target both the original COVID-19 strain and certain omicron subvariants. The recommendation followed the FDA’s emergency use authorization of the bivalent booster vaccine on August 31.
Unlike the original booster, now called the “monovalent” booster, which was formulated with spike protein components from the original COVID-19 strain, the bivalent booster includes spike protein components from both the original strain and the currently dominant omicron subvariants BA.4 and BA.5.
All individuals age 12 or older are eligible for a bivalent booster dose two months after their last primary series dose (i.e., two months after receiving a second dose of the Pfizer or Moderna vaccine or one dose of the J&J vaccine). Those who previously received monovalent booster doses are eligible two months after the last monovalent booster dose. This updated booster recommendation replaces the prior booster recommendation for individuals age 12 or older. Currently, the Pfizer bivalent booster is authorized for persons age 12 or older, and the Moderna bivalent booster is authorized for persons age 18 or older. Children ages 5 to 11 who received the Pfizer vaccine for their primary series will continue to receive the Pfizer monovalent boosters.
According to the CDC, people who recently had COVID-19 may consider delaying their next vaccine dose by three months from the date their symptoms started — or, if they had no symptoms, from the date of their first positive test. Studies suggest that increased time between infection and vaccination may result in an improved immune response to vaccination.
Given the variety of vaccines available for primary and booster series, we have developed the following graphics to help everyone navigate the various recommended vaccination schedules, including those who are immunocompromised.
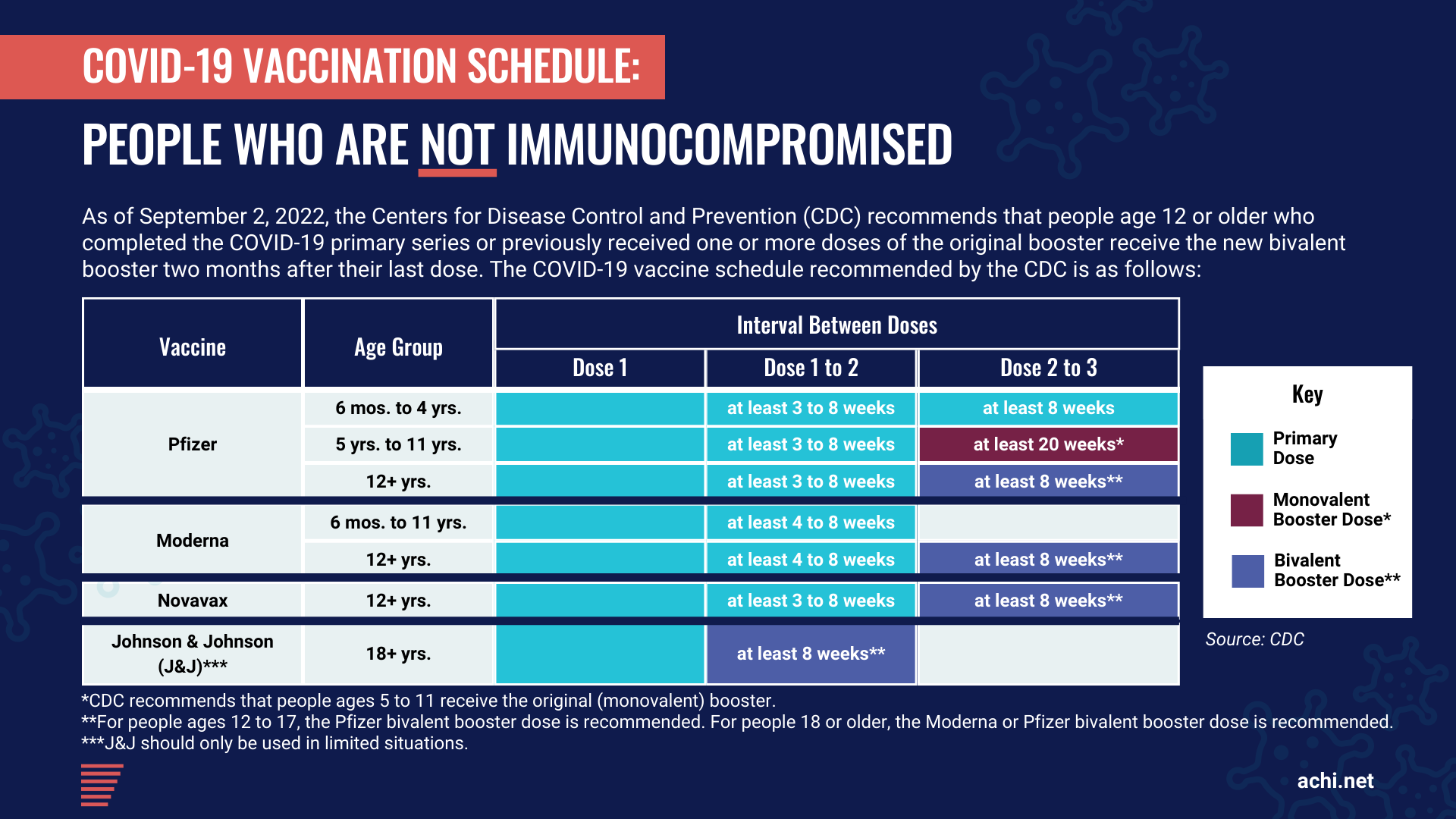
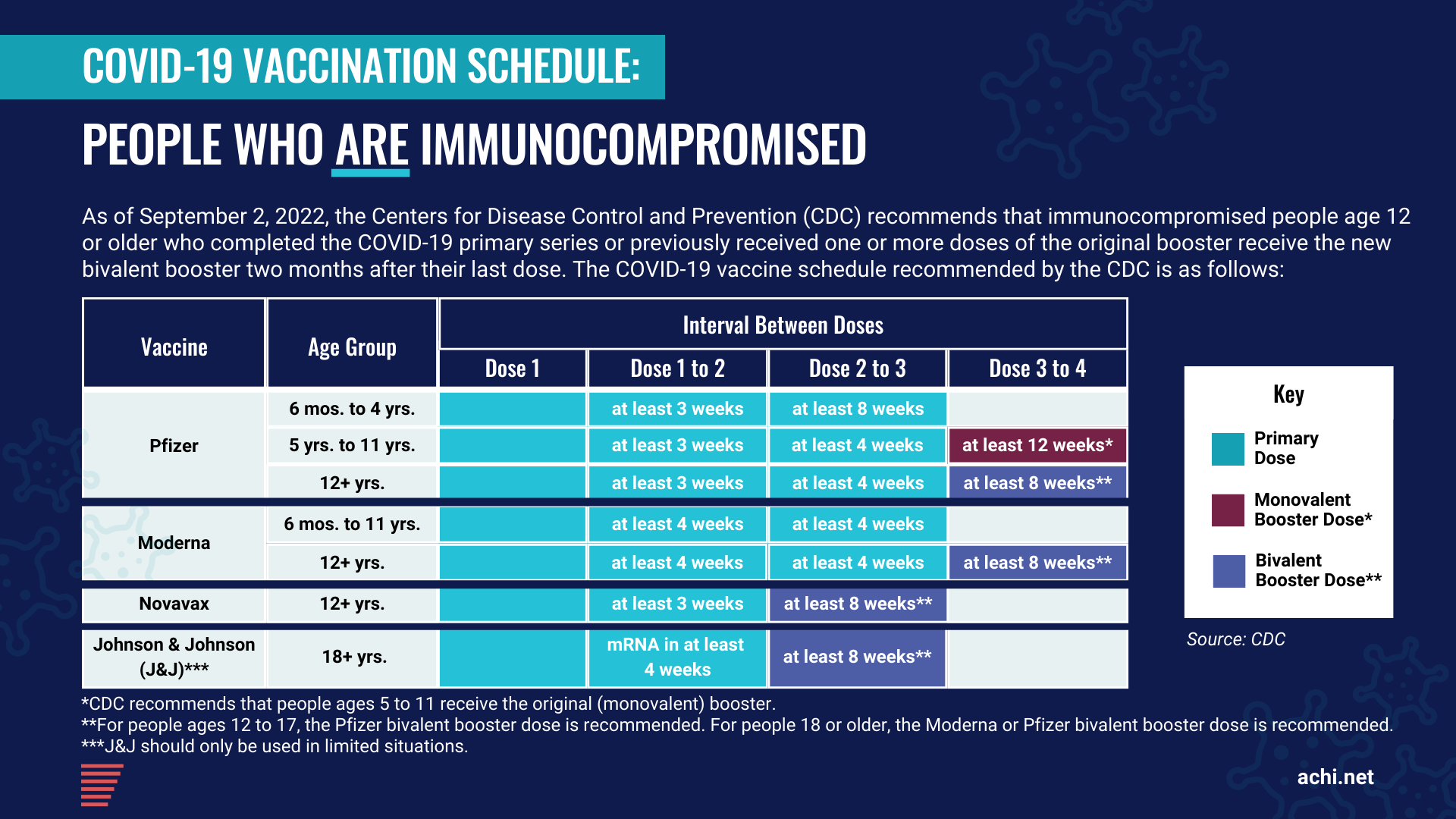
For the most current information, visit the CDC’s website.