COVID-19 in Arkansas
COVID-19 continues to affect people in our state. ACHI will provide updates and insights on emerging and related issues.
Last Updated: July 26, 2024
What To Know About The Latest COVID-19 Vaccine
The Food and Drug Administration and the Centers for Disease Control and Prevention approved new vaccines targeting more recent variants of the virus that causes COVID-19.
The CDC recommends everyone 6 months and older get an updated COVID-19 vaccine to protect against the potentially serious outcomes of COVID-19 illness this fall and winter. The updated vaccines from Pfizer and Moderna are currently available in many pharmacies around the state. We recommend that you check with your pharmacy for availability.
Vaccination remains the best protection against COVID-related hospitalization and death. Vaccination also reduces your chance of suffering the effects of long COVID, which can develop during or following acute infection and last for an extended duration. If you have not received the latest COVID-19 vaccine, get the updated shot to protect yourself, your loved ones, and vulnerable members of your community.
According to the CDC, the virus that causes COVID-19 is always changing, and protection from COVID-19 vaccines declines over time. Receiving an updated COVID-19 vaccine can restore protection and provide enhanced protection against the variants currently responsible for most infections and hospitalizations in the United States. In February 2024, the CDC reported that “adults with healthy immune systems who received an updated COVID-19 vaccine were about 50% less likely to visit an ED, urgent care, or be hospitalized with COVID-19 compared with those who didn’t.” To date, hundreds of millions of people have safely received a COVID-19 vaccine under the most intense safety monitoring in U.S. history.
For people with health insurance, most plans will cover the COVID-19 vaccine at no cost to you. People who don’t have health insurance or have health plans that do not cover the cost can get a free vaccine from local health units. More information is available here.
Another COVID-19 vaccine from Novavax has been authorized by the FDA under Emergency Use Authorization (EUA) and may be available at local pharmacies. For more information on the vaccine, go here: https://ir.novavax.com/press-releases/2023-10-03-Novavax-2023-2024-COVID-19-Vaccine-Now-Authorized-and-Recommended-for-Use-in-the-U-S
Current Status
As the availability of COVID-19 case reporting has decreased, many public health experts have pointed to hospitalizations as a more reliable COVID-19 indicator at this stage in the pandemic. To help you stay on top of the latest COVID-19 trends, ACHI is tracking the current status in Arkansas.
According to the Centers for Disease Control and Prevention, Arkansas had 46 new hospital admissions during the week ending April 27. This is down 8% from the week prior.
The CDC also tracks variants and sub-variants of the virus that causes COVID-19 and provides estimates of variant proportions, regionally and nationally. For the latest results of the CDC’s variant surveillance, see the data below or visit its COVID Data Tracker page.
CDC Variant Tracker
The CDC is monitoring the evolution of the virus that causes COVID-19. Some evolutionary lineages include key variations that may reduce the effectiveness of some treatments or increase the virus’ ability to spread.
The diagram below shows how the lineages are related to each other. For more, visit cov-lineages.org or the CDC’s COVID Data Tracker
Combating COVID-19 Misinformation
Amid evolving COVID-19 variants and the unknown impact of long COVID on millions of Americans and tens of thousands of Arkansans, ACHI is redoubling its commitment to combating misinformation regarding COVID-19 treatment and prevention.
For Trustworthy COVID-19 Treatment and Prevention Information:
- Food and Drug Administration
- The FDA has authorized a medicine — Pemgarda (pemivibart) — intended to protect certain immunocompromised people against COVID-19.
- National Institutes of Health (NIH) Advisory COVID-19 Treatment Panel
- The panel of health experts and physicians publishes updates on treatment of COVID-19.
- Currently, the panel recommends the following two therapies as preferred treatments for COVID-19:
- Paxlovid (granted full approval by the Food and Drug Administration in May 2023)
- Remdesivir
How to Identify Misinformation:
To combat misinformation, one must first learn how to spot it. Here are a few recommendations that may help you recognize COVID-19 treatment misinformation online:
- Determine the source of the information.
- Don’t equate likes and shares on social media posts with credibility.
- Ask yourself: What’s behind this information? What’s the evidence? What are other sources saying?
- Use authoritative sources.
- Verify the trustworthiness of the source:
- ICANN Lookup (allows you to see registration information for the website)
- Double-check the information on fact-checking sites:
- Verify the trustworthiness of the source:
Helpful Links and Additional Sources:
- Community Toolkit for Addressing Health Misinformation — U.S. Surgeon General
- Infographic on Steps to Spot Fake News — International Federation of Library Associates and Institutions
- 5 Ways to Spot Misinformation and Disinformation Online — Syracuse University School of Information Studies
- 10 Ways to Spot Disinformation on the Internet — AARP
- Social Media and Medical Misinformation: Confronting New Variants of an Old Pattern — Journal of the American Medical Association
- Evidence and Health: What Does the Future Hold? — Robert Wood Johnson Foundation
- AMA Adopts New Policy Aimed at Addressing Public Health Disinformation — American Medical Association
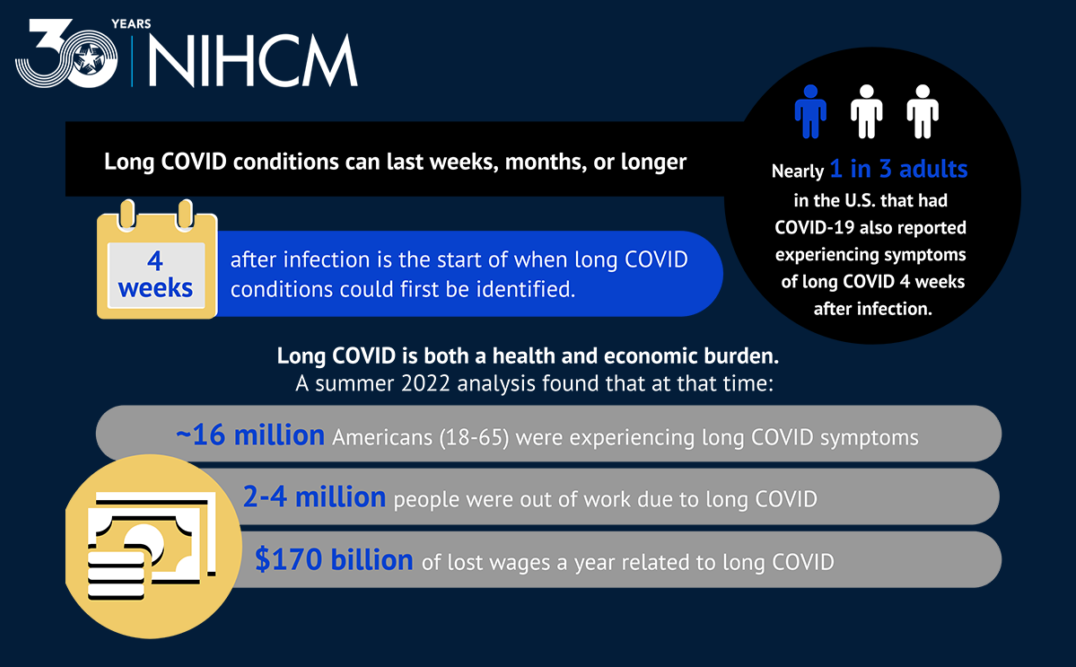
Source: “Long COVID: What We Know” – The National Institute for Health Care Management Foundation. February 2, 2023.
Some individuals who have been infected with the virus that causes COVID-19 can experience a wide range of ongoing health problems or long-term effects, commonly called long COVID. Anyone who has been infected with the virus can experience long COVID, including some people who never tested positive or knew they were infected. For more information about long COVID, a list of possible symptoms, and guidance for people dealing with long COVID, visit the CDC’s website.
COVID-19 Disparities and Long COVID Webinar

COVID-19 Disparities and Long COVID Podcast
Testing Information
When to test:
- COVID-19 and the flu have some symptoms in common. The FDA has authorized an over-the-counter, at-home test that can differentiate between the two illnesses.
- If you have symptoms or have been exposed (or potentially exposed) to an individual with COVID-19 you should self-test or get tested at a healthcare facility.
- Because you can be infected without symptoms or a known exposure to someone with COVID-19, using a self-test before gathering with others, either within your household or outside of your household, can inform you about your risk of spreading COVID-19 to others.
- A negative result means that the test did not detect the virus and that you may not have an infection, but it does not rule out infection, especially when self-testing. Repeating an at-home test within a few days, with at least 24 hours between tests, will increase the confidence that you are not infected.
Where to get tested or obtain at-home tests:
- Use the CDC’s COVID-19 Testing Locator to search for free COVID-19 testing sites near you. Testing sites could include local pharmacies, commercial laboratories, community sites, or retail locations.
- Free or low-cost at-home tests may be available at Local Health Units or local pharmacies.
- Commercial insurance carriers may pay or reimburse for at-home and lab tests, although you may be subject to cost-sharing (check with your insurance provider for conditions).
- All U.S. households are eligible to order four free at-home COVID-19 tests from covid.gov/tests.
How to perform a COVID-19 self-test:
- Always carefully review the instructions that come with your home test before getting started. There are many different manufacturers of home tests and the instructions may vary.
- Watch a demonstration on how to use one common at-home test.
- The CDC has put together a detailed page about at-home COVID-19 self-tests on their website.
What to do if your test result is positive:
- You should stay home or isolate for five days, if possible. If you are asymptomatic after five days and test negative, you may return to work, but you should continue to wear high quality masks through day 10 (N95s and KN95s offer better protection than cloth masks).
- Tell a healthcare provider about your positive test result and stay in contact with them.
- If your illness becomes severe, seek medical attention.
- If you have an emergency warning sign (including trouble breathing), seek emergency medical care immediately.
- Tell your close contacts that they may have been exposed to the virus that causes COVID-19. A person with COVID-19 can begin spreading it two days before they have any symptoms or test positive. By informing your close contacts that they may have been exposed, they can test at home and help protect their family and others.
- If you think your positive test result may be incorrect, contact a healthcare provider to determine whether or not additional testing is necessary.
- For more information about what to do if you test positive, refer to the CDC’s detailed guidance.
COVID-19-Related Blog Posts From Our Team
Defining COVID-19 Terms
As the pandemic continues to unfold, it can be difficult to keep up with emerging information about COVID-19, especially if you are unfamiliar with some of the terminology. In the early stages of this evolving health crisis, we launched a series of blog posts explaining key terms and phrases used by public health officials in discussions about this new disease. Check out some recent posts below, and see 30 Terms and Phrases Used by Public Health Officials When Talking About COVID-19 for a roundup of terms we’ve defined.
Excess Deaths
Excess deaths are defined as the difference between the observed number of deaths during a specific time frame and the expected number of deaths during the same period. ACHI has included excess death data resources on our website, including an interactive dashboard of excess deaths associated with COVID-19 developed by The Centers for Disease Control and Prevention (CDC). Read More
Clinical Trial
Clinical trials are a vital component of the development process for medications, vaccines, and other medical treatments and preventatives, including those for COVID-19. Medication combinations and new uses for current medications may also be evaluated in a clinical trial. A clinical trial is a type of study in which researchers test novel approaches to prevent, detect, or treat disease. Read More
Endemic
An endemic disease is one that is commonly found in a specific population or region. Endemic diseases are different from epidemics and pandemics, which are outbreaks of a disease that continue to spread to other regions (or globally in the case of a pandemic). An example of an endemic virus is influenza, better known as the flu. Read More

Helpful Links and Numbers
The Arkansas Department of Health (ADH) is tracking statewide cases, and more information can be found here, and en espanol.
Arkansas hotline for information about COVID-19 vaccination, including help scheduling appointments: 1-800-985-6030. The hotline is available from 8 a.m. to 6 p.m., Monday through Friday.
The CDC posts regular online updates with latest guidelines and information on COVID-19.
ADH: During normal business hours (8:00 a.m. – 4:30 p.m.), urgent and non-urgent calls, please dial 1-800-803-7847 or email ADH.CoronaVirus@arkansas.gov. After normal business hours and weekend calls, needing immediate response, please call 1-800-554-5738.
UAMS: Click here for screening information, including online, phone, and in-person screenings. Their COVID-19 Hotline is 1-800-632-4502.
Arkansas Children’s Hospital (ACH): For children younger than 18 years old, call 1-800-743-3616. Nursing staff will be available for questions and phone screenings 24 hours a day, seven days a week. Click here for more information from ACH.
CDC Mask Guidelines: Click here to view the mask guidelines from the CDC
COVID-19 Testing in Arkansas: Use the CDC’s testing locator to search for the nearest no-cost testing location. The U.S. Department of Health and Human Services (HHS) also provides information on how and where to get tested.
The CDC has recommendations for improving ventilation in homes and buildings to reduce the spread of COVID-19.