
Weight-loss drugs are surging in popularity, and patients being treated for weight issues may receive prescriptions for any of several newly approved medications. This is the first of a series of blog posts that will explore popular weight-loss drugs; we begin with a look at the active ingredients in the drugs and how they work.
Active Ingredients
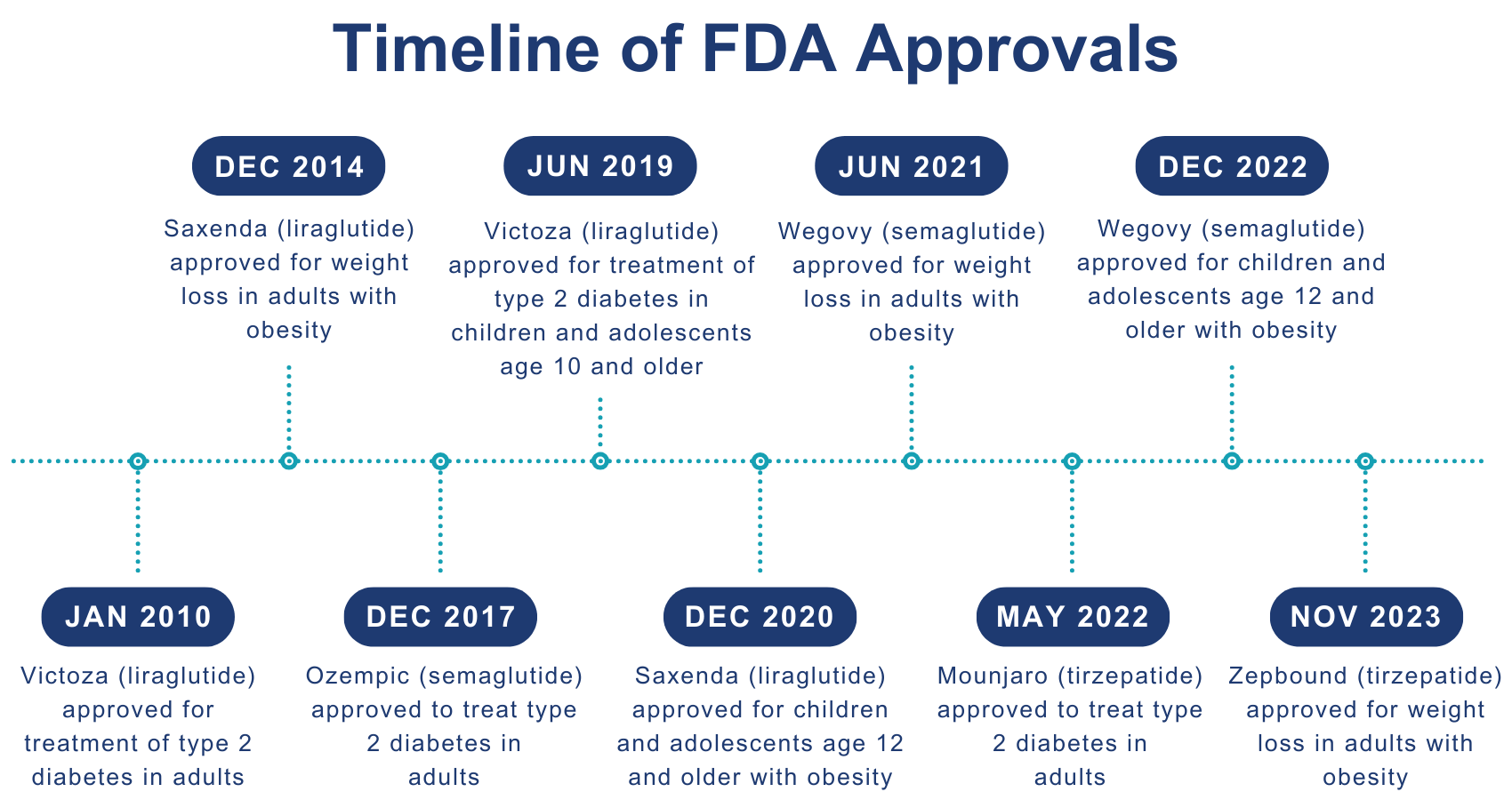
Zepbound is the latest new drug showing early promise in addressing challenges with weight loss. Manufactured by Eli Lilly, Zepbound was approved by the federal Food and Drug Administration (FDA) on Nov. 8, 2023, joining Wegovy and Saxenda in competing for market share in the category of drugs approved specifically for weight loss.
Tirzepatide, the active ingredient in Zepbound, is also the active ingredient in Mounjaro, approved in 2022 by the FDA to manage type 2 diabetes. Tirzepatide mimics hormones that are naturally produced in the gastrointestinal tract and help regulate blood sugar levels, decrease appetite, and slow the rate at which the stomach empties.
Zepbound’s approval is limited to adults who are obese, defined as having a body mass index (BMI) of 30 kg/m2 or greater; or adults who are overweight (BMI of 27 kg/m2 or greater) and who have other health problems such as high blood pressure or high cholesterol. Zepbound is not currently approved by the FDA for weight loss in children or adolescents.
Semaglutide was originally approved by the FDA in 2017 under the name Ozempic to treat adults with type 2 diabetes. It works similarly to tirzepatide, although it mimics only one type of hormone, whereas tirzepatide mimics two different types of hormone; some research suggests tirzepatide may be more effective for weight management than semaglutide.
Semaglutide is also the active ingredient in Wegovy, which was approved by the FDA in 2021 for long-term weight management for adults who are obese or overweight and have at least one weight-related condition such as high blood pressure, type 2 diabetes, or high cholesterol. In 2022, the FDA approved use of Wegovy, along with diet and exercise, for children and adolescents with a BMI greater than or equal to the 95th percentile — i.e., higher than 95% of people in that age group.
Liraglutide, the active ingredient in Saxenda and Victoza, mimics the same hormone as semaglutide. Liraglutide was approved by the FDA under the name Victoza in 2010 to treat adults with type 2 diabetes and as Saxenda in 2014 for weight loss. Victoza was approved by the FDA in 2019, for children and adolescents age 10 or older with type 2 diabetes. Saxenda was approved in 2020 for children and adolescents age 12 or older with obesity.
Effects and Side Effects
Besides weight loss and type 2 diabetes management, the purported benefits of these new weight-loss medications range from reducing the need for sleep apnea medication to having profound positive effects on heart health, including reducing the risk of major cardiac events like strokes, heart attacks, and death by as much as 20%. Some studies suggest that these medications may help curb alcohol and opioid addiction.
A study published in the New England Journal of Medicine (NEJM) in 2021 found that Wegovy led to a 15% reduction in body weight, on average. A study at Penn Medicine, funded by Novo Nordisk, found that participants taking semaglutide lost an average of 16% of their starting weight and more than one-third lost 20% or more of their baseline weight during the 68-week study. Another study published in the NEJM, also supported by Novo Nordisk, showed that patients with preexisting cardiovascular disease who were obese or overweight but did not have diabetes were less likely to experience death from cardiovascular causes, non-fatal myocardial infarctions, or non-fatal strokes while taking semaglutide.
Evidence thus far shows that these drugs can be effective but also suggests that long-term treatment will be necessary to maintain weight loss. In a study funded by Novo Nordisk, after discontinuing treatment with semaglutide, gains in cardiometabolic health (such as improved blood pressure, cholesterol levels, and blood sugar) reverted back toward initial levels, and participants regained two-thirds of the weight they had lost, suggesting the drugs may need to be taken long-term, possibly for the life of the patient. Similar results were found with tirzepatide.
These drugs can also have troublesome side effects, leading many to discontinue treatment. The most common complaint among people using medications like Ozempic, Wegovy, Mounjaro, and Zepbound is gastrointestinal issues such as nausea, vomiting, diarrhea, or constipation. Other side effects include dizziness, fatigue, and headaches. In some cases, more severe side effects include gastroparesis, pancreatitis, or bowel obstructions. More evidence is needed to understand long-term effects that remain unknown.
In our next post in this series, we will explore the use of these drugs for children and adolescents who are obese or overweight.






