
Author
Elizabeth (Izzy) Montgomery, MPA
Policy Analyst
Contact
ACHI Communications
501-526-2244
jlyon@achi.net
State Medicaid programs are increasingly providing coverage for weight-loss drugs, including glucagon-like peptide-1 (GLP-1) drugs, according to a recent report from KFF.
The Food and Drug Administration (FDA) has approved three GLP-1s for obesity-related treatment, including Saxenda (liraglutide), Wegovy (semaglutide), and Zepbound (tirzepatide). Initially, GLP-1s were developed to treat type 2 diabetes and were marketed for that purpose under brand names such as Ozempic (semaglutide), Rybelsus (semaglutide), Victoza (liraglutide), and Mounjaro (tirzepatide).
State Medicaid programs have the authority to determine whether they will cover weight-loss drugs. State participation in the Medicaid Drug Rebate Program requires coverage of nearly all FDA-approved drugs for medically accepted indications, but weight-loss drugs are included in a small group of drugs that can be excluded from coverage.
State Medicaid Coverage of GLP-1s for Obesity Treatment (as of August 2024)
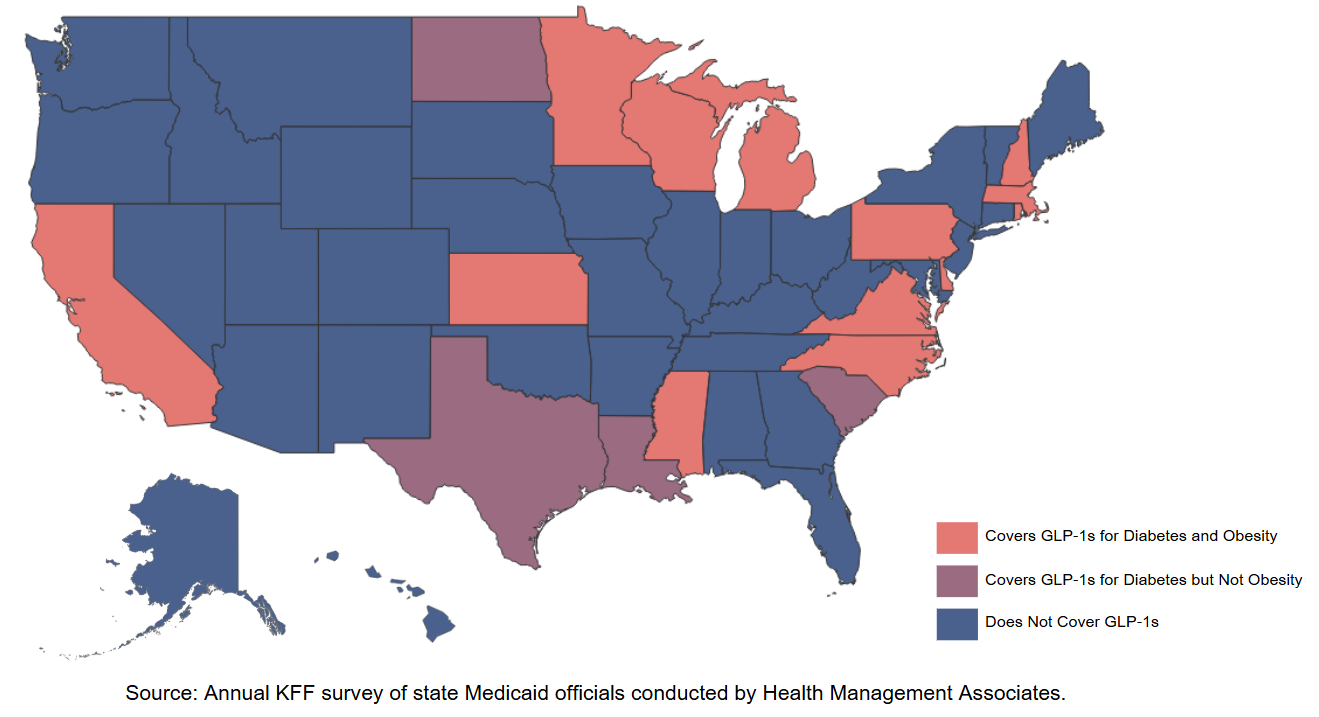
KFF found that as of August 2024, 13 state Medicaid programs covered GLP-1s for obesity treatment within their fee-for-service programs. As of a July 2024 survey, among the states providing coverage, 11 required prior authorization, and 11 had policies tying coverage to body mass indices. Eleven states covered all three GLP-1s approved for obesity treatment. Two states, Louisiana and South Carolina, provided coverage for one weight-loss drug that is not a GLP-1, orlistat.
According to KFF, both GLP-1 prescriptions and gross spending (Medicaid spending before drug rebates are applied) on GLP-1s have increased rapidly within state Medicaid programs in the past few years. From 2019 to 2023, there was a 400% increase in prescriptions and a 500% increase in gross spending. In 2023, gross Medicaid spending was approximately $3.9 billion for these drugs, with nearly $2 billion in spending on Ozempic alone. Notably, gross spending does not account for drug rebates, which are paid by drug manufacturers to states and are shared between the states and the federal government to offset the overall cost of prescription drugs under states’ Medicaid programs.
Recently, an Arkansas Legislative Council subcommittee recommended that the state’s public school and state employee health plan, which is overseen by the Employee Benefits Division, implement a diabetes management program that includes control over GLP-1 drug costs. Currently, the plan covers GLP-1 drugs for diabetes treatment but not for obesity.
For more on weight-loss drugs, see our blog series.






